UNIQUE MECHANISM
OF ACTION
NEMLUVIO is the first and only neuroimmune-targeted treatment to directly block IL‑31RA1,2
NEMLUVIO® blocks IL-31 signaling across prurigo nodularis and atopic dermatitis disease processes1,2
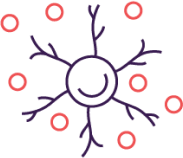
Mechanism of disease
Unlike other cytokines, overexpression of IL-31 and IL-31RA has been shown to be a direct driver of itch and more. IL‑31 binds to receptors across multiple cell types to drive PN and AD1,2
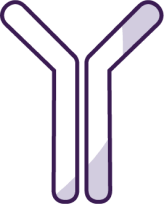
Mechanism of action
NEMLUVIO targets an IL-31 receptor, blocking signaling that drives itch, inflammation, skin barrier dysfunction, and fibrosis1,2
WATCH THE VIDEO BELOW TO SEE HOW NEMLUVIO WORKS
NEMLUVIO is a humanized IgG2 monoclonal antibody.1
IL-31, the neuroimmune cytokine, directly drives disease processes by binding to IL-31 receptor alpha to: activate sensory nerves in the skin and on the dorsal root ganglion to amplify the itch signal to the brain, which compels the patient to scratch, interact with multiple cell types to drive TH2 and non-TH2 inflammation, interfere with keratinocyte differentiation that leads to skin barrier dysfunction, stimulate fibroblasts to induce collagen deposition and tissue remodeling that result in fibrosis.
NEMLUVIO is the first and only neuroimmune treatment that targets IL-31 signaling to work across four disease processes: blocking signals in the sensory nerves and uniquely on the dorsal root ganglion to directly suppress itch, blocking IL-31 receptor alpha to disrupt a key cause of harmful inflammation in the immune system, blocking the IL-31RA on keratinocytes to normalize the skin barrier, and finally, NEMLUVIO blocks the IL-31RA on fibroblasts to inhibit pro-fibrotic and inflammatory responses to heal nodules.
Fast Itch Relief8
See how NEMLUVIO significantly improved itch in both pivotal and long-term studies8
Patient Profiles
Take a closer look at the types of patients who may benefit from NEMLUVIO
Sign up now for more information about NEMLUVIO for PN
AD=atopic dermatitis; IL-31=interleukin 31; IL-31RA=interleukin-31 receptor alpha; PN=prurigo nodularis.
References: 1. NEMLUVIO (nemolizumab-ilto) injection 30 mg Prescribing Information. Dallas, TX: Galderma Laboratories, L.P. 2. Nemmer JM, Kuchner M, Datsi A, et al. Interleukin-31 signaling bridges the gap between immune cells, the nervous system and epithelial tissues. Front Med (Lausanne). 2021;8:639097. doi:10.3389/fmed.2021.639097 3. Dubin C, Del Duca E, Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. 2021;17(8):835-852. doi:10.1080/1744666X.2021.1940962 4. Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138(2):500-508.e24. doi:10.1016/j.jaci.2016.02.020 5. Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front Immunol. 2019;10:1383. doi:10.3389/fimmu.2019.01383 6. Hänel KH, Pfaff CM, Cornelissen C, et al. Control of the physical and antimicrobial skin barrier by an IL-31-IL-1 signaling network. J Immunol. 2016;196(8):3233-3244. doi:10.4049/jimmunol.1402943 7. Mousa A, Bakhiet M. Role of cytokine signaling during nervous system development. Int J Mol Sci. 2013;14(7):13931-13957. doi:10.3390/ijms140713931 8. Galderma Laboratories, L.P.; data on file.