LASTING SKIN HEALING
NEMLUVIO heals nodules to clear the skin in PN1
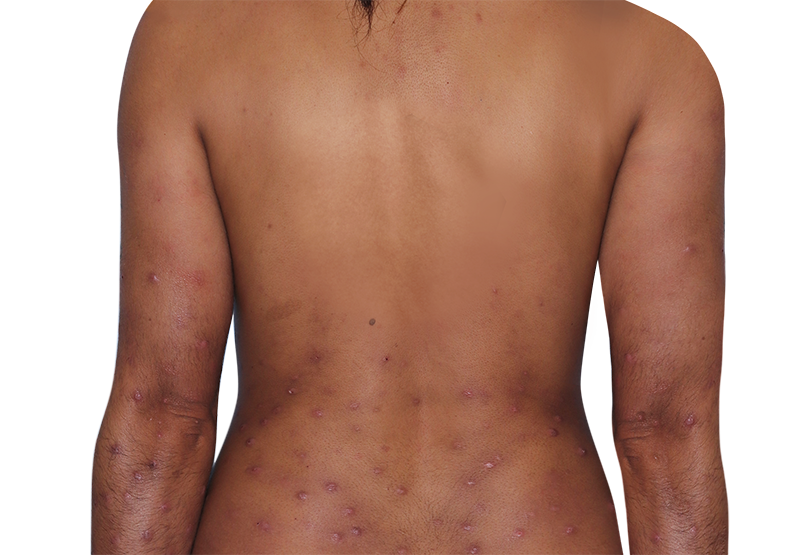
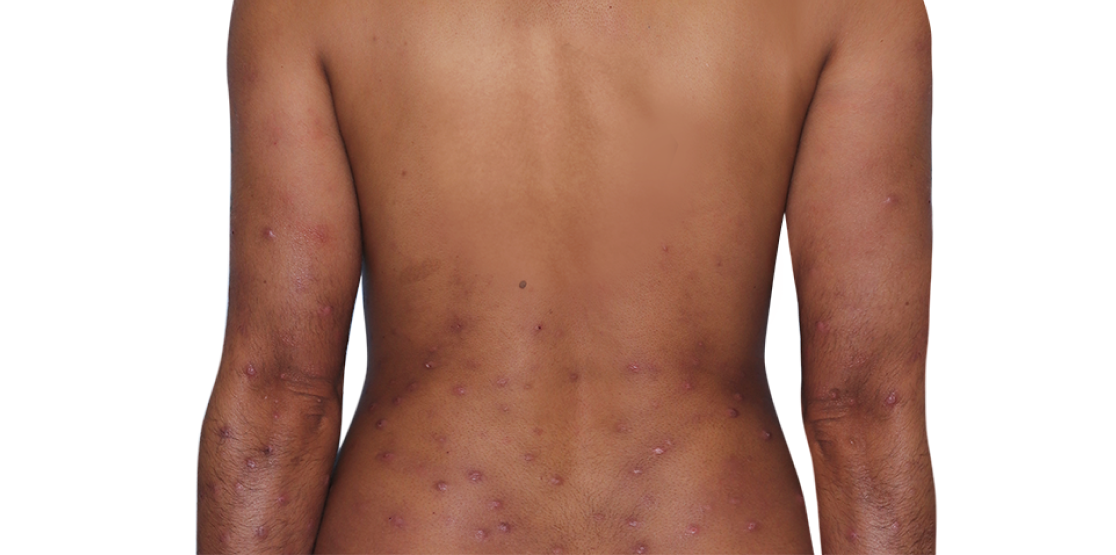


Baseline
Week 16
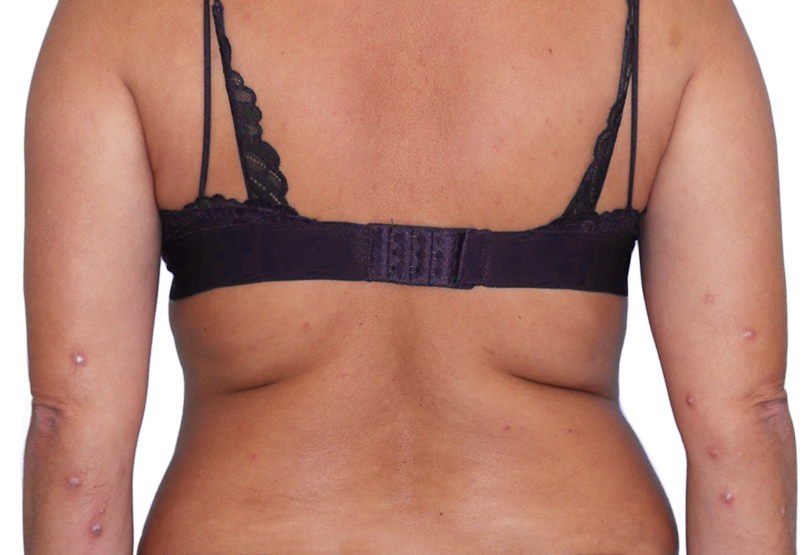
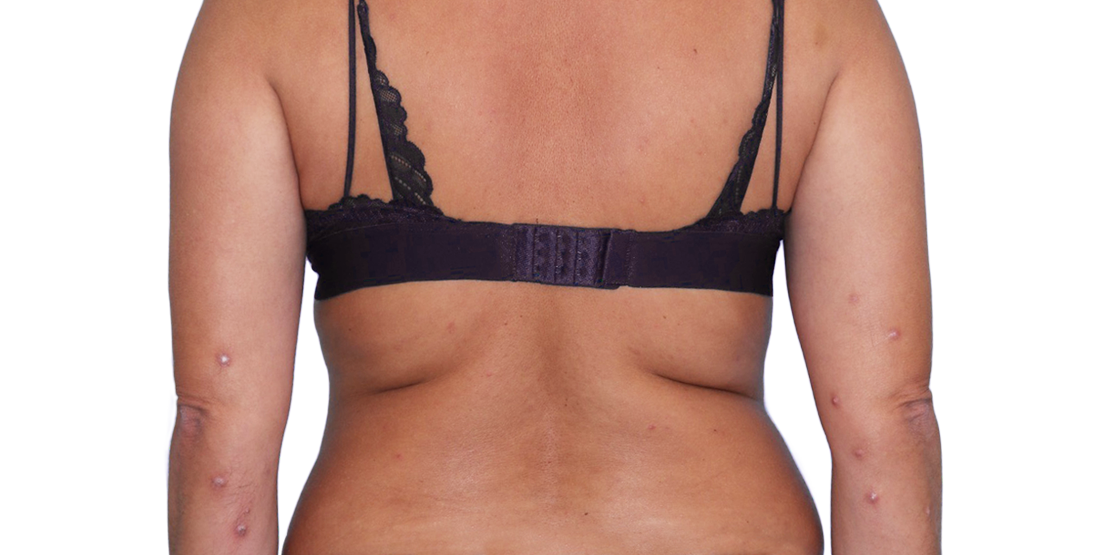
31-YEAR-OLD FEMALE.
Actual patient.
Individual results
may vary.
31-YEAR-OLD FEMALE.
Actual patient. Individual results may vary.
Photo altered to remove identifying marks.
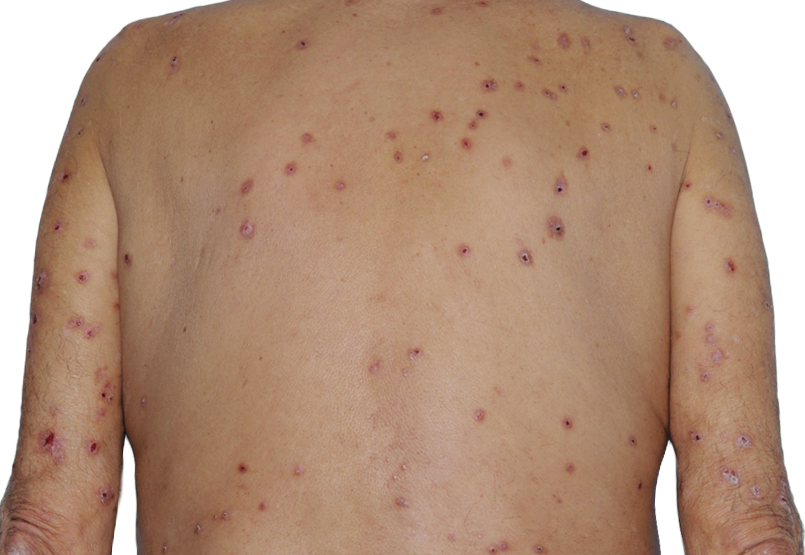
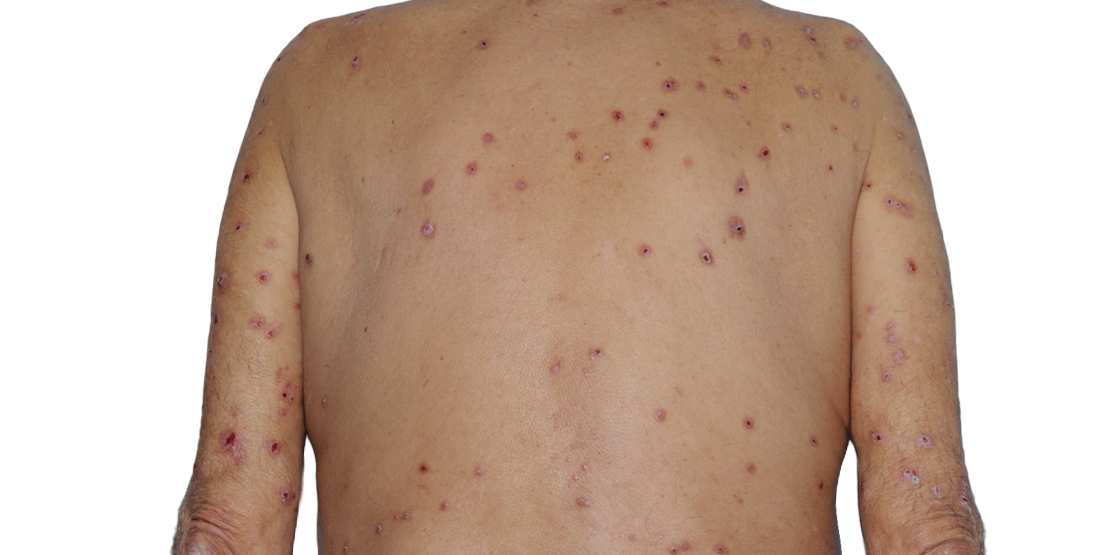
Baseline
Week 16
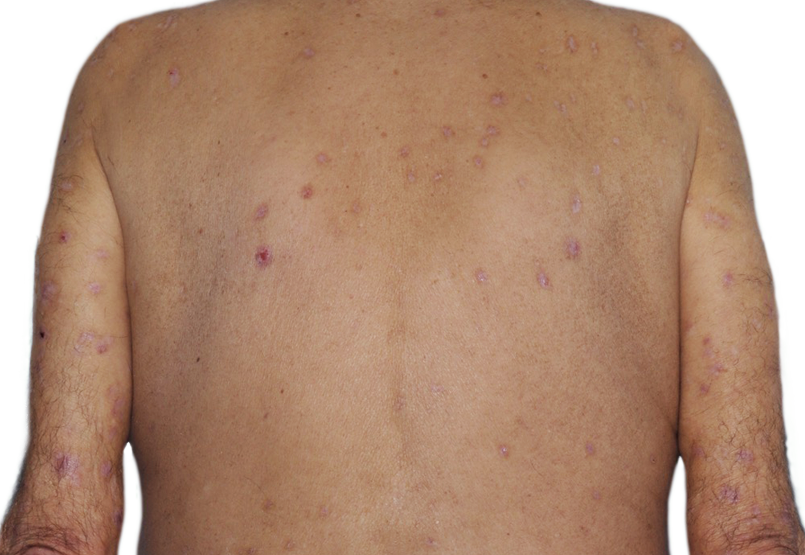
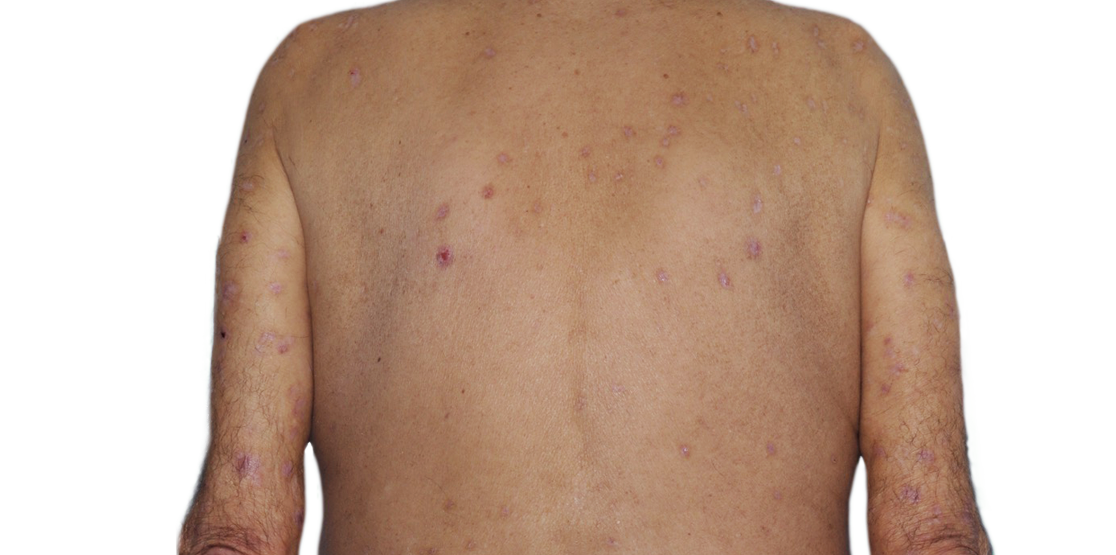
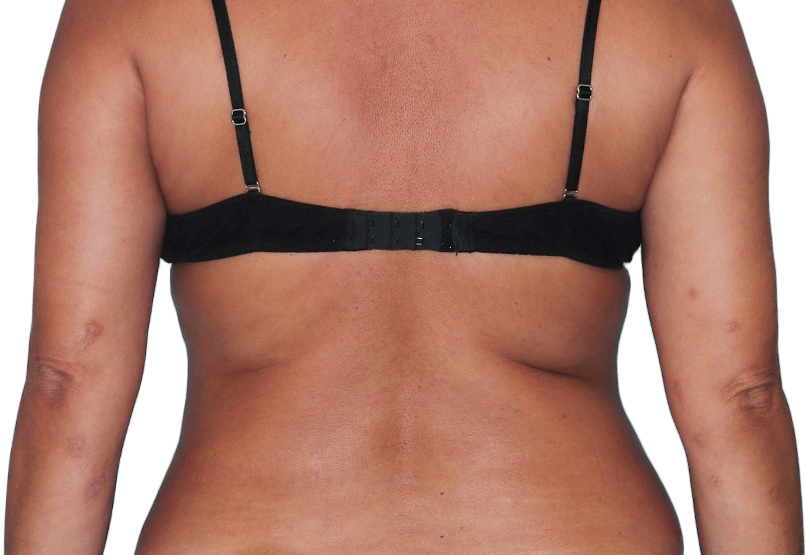
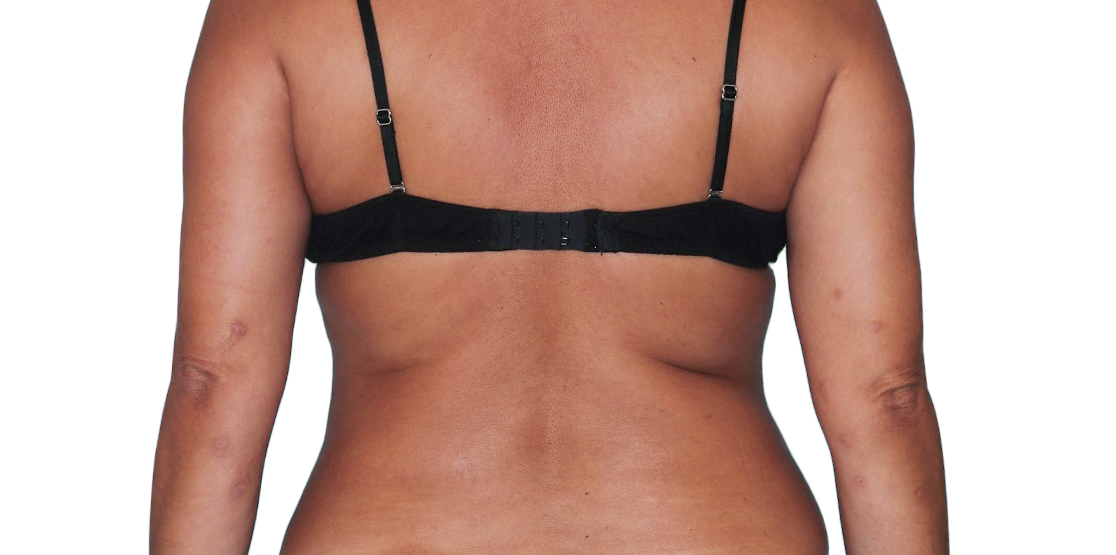
73-YEAR-OLD MALE.
Actual patient.
Individual results
may vary.*
73-YEAR-OLD MALE.
Actual Patient. Individual results may vary.*
Baseline
Week 16
43-YEAR-OLD
FEMALE.
Actual patient.
Individual results
may vary.
43-YEAR-OLD FEMALE.
Actual patient. Individual results may vary.

31-year-old female.
73-year-old male.

43-year-old female.
*Baseline IGA score was 4, and at Week 16, it had improved to 2.5
Previous
Next
of nodules healed in more than half of patients taking NEMLUVIO by Week 16 vs 17% with placebo (P<0.0001)1*
Data above from OLYMPIA 2. In OLYMPIA 1, 41% of patients taking NEMLUVIO achieved >75% nodule healing* vs 12% with placebo at Week 16.1,2
8 out of 10 patients saw >75% of nodules healed with over a year of treatment3
Data from observed cases in OLYMPIA 2 treat-through population, interim analysis of the OLYMPIA LTE.
No imputations for missing data. In the full population, 79% of patients (n=313) achieved PAS item 5b improvement at Week 52. The OLYMPIA LTE is an ongoing prospective, multicenter, single-arm, open-label study up to 184 weeks.3,4
Sleep Improvement1
Patients saw meaningful improvements in their sleep while taking NEMLUVIO1
Favorable Safety Profile5
NEMLUVIO offers a favorable safety profile and requires no preliminary lab evaluations or ongoing lab monitoring5
Sign up now for more information about NEMLUVIO for PN
Response defined as PAS item 5b (76% to 100% healed prurigo lesions).1
IGA=Investigator Global Assessment; LTE=long-term extension; PAS=Prurigo Activity Score; PN=prurigo nodularis; Q4W=every 4 weeks.
References: 1. Galderma Laboratories, L.P.; data on file. Clinical Study Report RD.06.SRE.203065 [OLYMPIA 2]; September 2023. 2. Galderma Laboratories, L.P.; data on file. Clinical Study Report RD.06.SRE.202685 [OLYMPIA 1]; September 2023. 3. Galderma Laboratories, L.P.; data on file. 4. Galderma Laboratories, L.P.; data on file. Clinical Study Report RD.06.SIR.202699 [OLYMPIA LTE]; October 2023. 5. NEMLUVIO (nemolizumab-ilto) injection 30 mg Prescribing Information. Dallas, TX: Galderma Laboratories, L.P.