DOSING & ADMINISTRATION
NEMLUVIO offers Q4W dosing from the start1
LESS FREQUENT DOSING COMPARED TO THE OTHER BIOLOGIC FOR PN1,2
RECOMMENDED DOSING FOR PATIENTS 18 AND OLDER1
| Patient Weight | Initial Dose | Maintenance Dose (Q4W) |
| Less than 198 lbs (90 kg) | 2 injections | 1 injection |
| Greater than or equal to 198 lbs (90 kg) | 2 injections | 2 injections |
| Patient Weight | Initial Dose |
| Less than 198 lbs (90 kg) | 2 injections |
| Greater than or equal to 198 lbs (90 kg) | 2 injections |
| Patient Weight | Maintenance Dose (Q4W) |
| Less than 198 lbs (90 kg) | 1 injection |
| Greater than or equal to 198 lbs (90 kg) | 2 injections |
Each injection is 30 mg of NEMLUVIO®1
NEMLUVIO is uniquely designed for patient satisfaction
Room-temperature storage
for up to 90 days within the labeled expiration period1
>75% lower volume
than dupilumab
(0.49 mL vs 2 mL)1,2
Convenience
of a pre-filled
self-injectable pen1
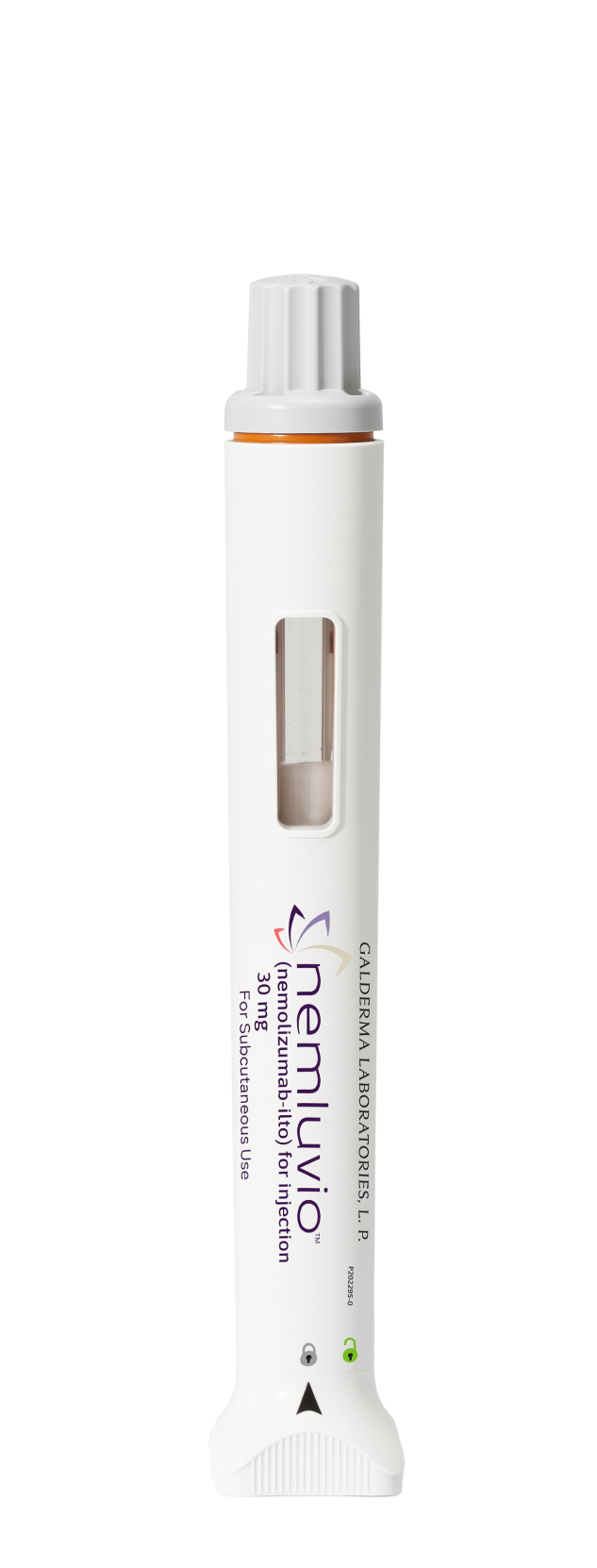
Administer NEMLUVIO seamlessly and safely

Unlock the auto-injector pen by turning the knob to the right—only once
Shake for 30 SECONDS in an upright position to reconstitute
Wait for 5 MINUTES until the bubbles have faded and the powder has dissolved completely
Twist the gray cap gently counterclockwise while holding upright until the orange needle guard pops up
Inject for 15 SECONDS with the pen flat on the skin

The NEMLUVIO Instructions for Use should be read carefully before using NEMLUVIO
Watch the NEMLUVIO Demo Kit Video to see how to successfully administer NEMLUVIO to your patients
Hi, and thanks for checking in on this practice session.
Meet John. He is going to show you how to practice with the NEMLUVIO Demonstration Pen—so that you can learn how to administer the injection to patients, and instruct able patients or caregivers to use the Pen at home.
The NEMLUVIO Demonstration Pen is for demonstration purposes only, and doesn’t contain medicine or a needle. The NEMLUVIO Demonstration Pen shouldn’t be distributed to patients. The actual NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen requires reconstitution of the medicine before it’s injected.
For important information, and storage information on the Demonstration Pen, read the instructions for NEMLUVIO Demonstration Pen. For directions on how to store, prepare, administer and dispose of the actual NEMLUVIO Pen, read the NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen Instructions for Use.
Before we start preparing the Demonstration Pen, let’s get familiar with its different parts. Remember, the actual NEMLUVIO Pen may look a little different.
Here’s how the Demonstration Pen looks before use: Starting at the top, here's the gray reset cap. The inspection window just below. And the activation knob at the bottom in the ‘Locked’ position, as shown by the lock icon.
After use, you’ll see these parts: The reset rod under the gray cap. The orange needle guard. The gray rod, and orange rod in the inspection window. And the activation knob at the bottom which, after use, will be in the ‘Unlocked’ position.
Now we can start preparing the NEMLUVIO Demonstration Pen, which is a three step process: First, hold the NEMLUVIO Demonstration Pen upright, and turn the activation knob to the right until it stops. Now, it will be in the ‘Unlocked’ position. Next, watch the inspection window until the gray rod stops moving. Lastly, when the gray rod has completely stopped moving, shake the NEMLUVIO Demonstration Pen up and down for 30 seconds. In the actual NEMLUVIO Pen, this simulates the process step for dissolving the medicine. For the actual NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen, we recommend waiting 5 minutes for the bubbles to decrease before injecting. If the medicine has not dissolved, instruct the patient to shake for another 30 seconds. It’s important to check to see that the dissolved medicine is clear, colorless, and does not contain particles before injecting. Once you complete the simulation on how to dissolve the drug, you’re ready to practice the injection.
Now let’s show your patient how to use it correctly. First, show your patient how to choose an injection site, using the chart included in the NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen Instructions for Use. If your patient will be self-injecting, they can inject the product in the abdomen, 2 inches (or 5 cm) away from their navel. Or, the upper thigh. If the injection will be given by a caregiver, they can place it in the patient’s abdomen, 2 inches (or 5 cm) away from the navel. The upper thigh. Or the outer upper arm. Make sure your patient cleans the injection site with an alcohol wipe before using the actual NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen. Once the injection site has been chosen, you can unscrew the gray cap off the NEMLUVIO Demonstration Pen. To do so, hold the NEMLUVIO Demonstration Pen upright. Unscrew the gray cap off, until the orange needle guard pops up. Then gently pull the cap off the orange needle guard. Instruct your patient to place the NEMLUVIO Demonstration Pen at a 90-degree angle on the injection site, so that the orange needle guard is flat against the skin. Then gently push the NEMLUVIO Demonstration Pen down—until the orange needle guard is completely pushed in, and you hear a ‘click’. With the actual NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen, this ‘click’ indicates that the injection has started. Hold the NEMLUVIO Demonstration Pen, and slowly count to 15. Then check the inspection window to make sure the orange rod, and the gray rod, have stopped moving. Once the orange and gray rods have stopped moving, lift the NEMLUVIO Demonstration Pen straight up from the skin. The orange needle guard will lock into place. When using the actual NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen, the orange needle guard will cover the needle.
After the demonstration, reset, and keep the NEMLUVIO Demonstration Pen before the next use. First, turn the activation knob to the left, to the ‘Locked’ (or original) position. Then replace the cap by pushing the reset rod all the way in while simultaneously slightly twisting the cap. You’re now ready to practice again. Remember, the actual NEMLUVIO Single-Dose Pre-Filled Dual-Chamber Pen should not be reset, but instead safely discarded. So, do not attempt to reset the actual NEMLUVIO Pen. And do not let your patient reset this Demonstration Pen. You can reset and reuse the Demonstration Pen for at least 120 cycles. And, you can come back to watch this video—or read the step-by-step instructions for NEMLUVIO Demonstration Pen—at any time.
For any questions, please reach out to your Galderma Representative. Thanks for watching.
Visit the NEMLUVIO Patient Site to watch an injection training video for self-administering patients
What patients should know before getting started
- Keep the NEMLUVIO pen and all medicines out of the reach of children3
- It is best to store the NEMLUVIO pen in the refrigerator between 36 °F and 46 °F (2 °C to 8 °C)3
- However, the NEMLUVIO pen can be stored at room temperature up to 77 °F (25 °C) for a single period up to 90 days3
- Take the NEMLUVIO pen out of the refrigerator and let it come to room temperature for 30 to 45 minutes before continuing3
- After the medicine has dissolved, it must be used within 4 hours. If you have not used it within 4 hours, dispose of it3
- Patients should mark their calendar ahead of time to remember when to take NEMLUVIO3
- The NEMLUVIO pen should not be used if it has been dropped on a hard surface or is damaged, cracked, or broken3
- The used NEMLUVIO pen should be disposed of right away after use in a sharps disposal container3
- Patients should be encouraged to complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to NEMLUVIO treatment initiation1
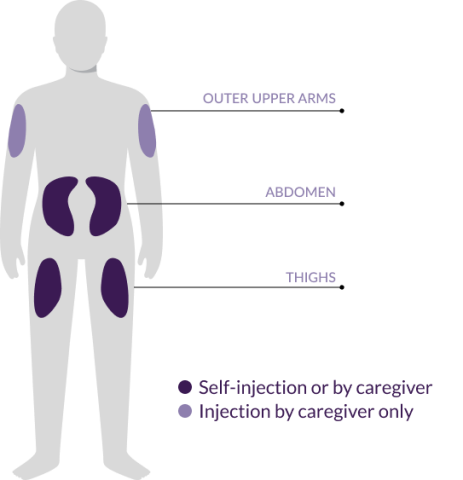
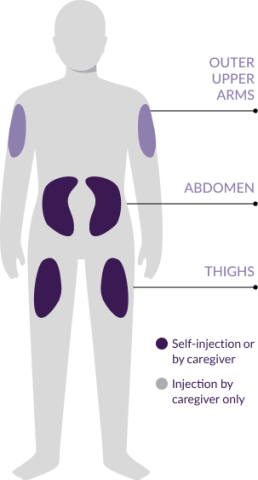
Where to inject
- Select the injection site using the following chart3
- Inject the pen in the abdomen (2 inches away from the navel), upper thigh, or the outer upper arm3
- Use a different injection site at least 1 inch away from the first injection site3

GPS (Galderma Patient Services) for NEMLUVIO™
Helps patients start and stay on treatment by providing access to assistance programs and adherence support
Patient and Practice Resources
Tools to help patients start and stay on treatment
Sign up now for more information about NEMLUVIO for PN
PN=prurigo nodularis; Q4W=every 4 weeks.
References: 1. NEMLUVIO (nemolizumab-ilto) injection 30 mg Prescribing Information. Dallas, TX: Galderma Laboratories, L.P. 2. DUPIXENT Prescribing Information. Sanofi and Regeneron Pharmaceuticals, Inc.; 2024. 3. NEMLUVIO (nemolizumab-ilto) injection 30 mg Instructions for Use. Dallas, TX: Galderma Laboratories, L.P.